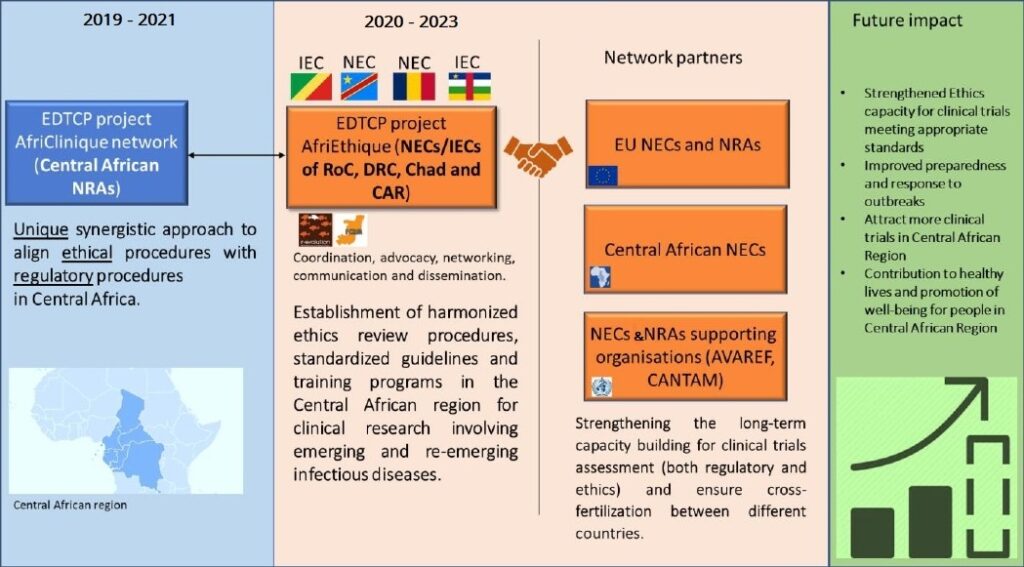
Despite being home to >16% of the world’s population, Africa currently only hosts <5% of the clinical studies that are executed worldwide. Especially the number of clinical trials in Central African countries such as Republic of the Congo (RoC), Central African Republic (CAR), Democratic Republic of Congo (DRC), and Chad are lagging, compared to other African regions. One important cause for this is the absence of proper ethics review capacity to ensure efficient and timely evaluation and approval of clinical research proposals.
Within this context, the following African and European institution developed and submitted a proposal to the European and Developing Countries Clinical Trials Partnership (EDCTP) for a research project focused on Central Africa ethics infrastructures and efficiency: Fondation Congolaise pour la Recherche Médicale (FCRM, Republic of Congo), ), Comité Scientifique de Validation des Protocoles et Résultats de Recherche en Santé at Institut Pasteur de Bangui (Central African Republic), Comité national d’ethique de la santé (CNES – Democratic Republic of Congo), Unité De Pharmacologie Clinique et Pharmacovigilance (UPC-PV, Democratic Republic of Congo), Ministère de l’enseignement supérieur, de la rechecher et de l’innovation, Comité National de Bioéthique du Tchad (CNBT, Chad) and the European not for profit organization R-Evolution Worldwide (R-EvoWW, UK and Italy).
The main aim of the AfriEthique project is to strengthen the Ethics review capacity for clinical research in DRC, CAR, Chad, and RoC, with a specific focus on emerging and re-emerging diseases. To achieve this, a sustainable collaborative network will be established connecting Central African National Ethics Committees (NECs), European NECs and NEC-supporting initiatives (e.g. AVAREF and CANTAM).
AfriEthique will also set in motion improvement and harmonization of ethics and regulatory capacity in Central African countries, by building a sustainable and collaborative network that connects European NECs and NRAs, Central African NECs and NRAs from the approved EDTCP ‘’AfriClinique’’ project.